Are you trying to separate delicate materials? Confused if molecular distillation is just another name for vacuum distillation? Let's clear this up.
No, molecular distillation is not exactly the same as vacuum distillation. It's a highly specialized type of short-path vacuum distillation. It's designed for purifying very sensitive, high molecular weight compounds that other methods can't handle well.

Understanding the difference is important. It can help you choose the right equipment for your specific needs. As a manufacturer with over 16 years of experience in laboratory instruments, especially extraction and distillation equipment, we want to help you understand these processes. Let's dive into what makes each unique.
What exactly is vacuum distillation then?
Do your heat-sensitive compounds degrade during normal distillation? Worried about losing valuable product? Vacuum distillation offers a gentler way.
Vacuum distillation is a process that lowers a liquid's boiling point by reducing the pressure inside the distillation apparatus. This allows for distillation at lower temperatures. This is key for protecting heat-sensitive substances from breaking down.
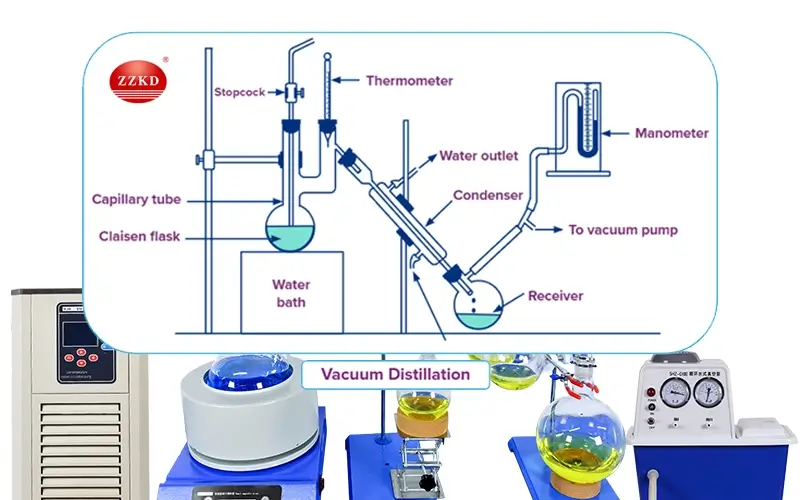
Vacuum distillation is a very useful technique we see in many labs and industries. The main idea is simple. Most liquids boil when their vapor pressure equals the pressure around them. If we lower the pressure around the liquid, it doesn't need to get as hot to boil. Think of it like trying to lift a heavy lid – it’s easier if someone helps reduce the weight pressing down. Our vacuum distillation units are designed to create this low-pressure environment reliably.
How Does It Work?
We use a vacuum pump to remove air and other gases from the distillation system. This reduces the overall pressure. With less pressure, the molecules in the liquid can escape into the gas phase more easily, and at a lower temperature. This is crucial for compounds that might decompose, oxidize, or undergo unwanted reactions if heated too much at normal atmospheric pressure. I've seen many clients successfully purify materials that would otherwise be lost using this method.
Common Applications:
Chemical Industry: Purifying solvents, separating isomers, or isolating compounds that have high boiling points.
Pharmaceuticals: Concentrating active pharmaceutical ingredients (APIs) that are thermally unstable.
Food and Beverage: Extracting essential oils or concentrating fruit juices without losing flavor or nutritional value due to heat.
Petrochemicals: Refining crude oil into various fractions.
While very effective for many situations, standard vacuum distillation still has limitations. For extremely large molecules or super sensitive ones, even the reduced temperature might not be enough, or the path the vapor travels might be too long. This is where we start looking at more specialized techniques.
So, how is molecular distillation different and special?
Need the highest purity for complex oils or extracts? Are traditional vacuum methods still falling short for your delicate materials? Molecular distillation provides an advanced solution.
Molecular distillation, often called short-path molecular distillation, operates at a much higher vacuum (extremely low pressure). It also features a very short distance, just a few centimeters, between the surface where the liquid evaporates and the surface where the vapor condenses.

This is where things get really interesting, and it's an area where our company, Zhengzhou Keda, has focused a lot of research and development. The "molecular" part of the name comes from the concept of the "mean free path." This is the average distance a molecule travels before it bumps into another molecule. In molecular distillation, we create such a high vacuum that the mean free path of the evaporating molecules becomes longer than the distance to the condenser surface. This is a game-changer.
Key Features That Make It Special:
Extremely High Vacuum: We're talking pressures typically below 0.001 mbar. This drastically lowers the boiling temperature, sometimes by hundreds of degrees Celsius. Our systems are built to achieve and maintain these deep vacuum levels.
Ultra-Short Path: The evaporator and condenser are very close. This means molecules, once evaporated, travel a very short distance directly to the condenser. There's minimal chance for them to collide with other molecules or hit hot surfaces again. This reduces thermal stress and prevents degradation.
Wiped Film Mechanism (Often): Many of our molecular distillation units use a wiped film system. Rotating wipers spread a very thin film of the feed material on the heated evaporation surface. This ensures uniform heating, rapid evaporation, and is great for viscous materials that wouldn't flow well otherwise.
Because of these features, molecular distillation can separate very large molecules (high molecular weight), like those found in oils, fats, waxes, and resins, that would simply cook or decompose in other distillation setups. It's a gentle but powerful purification technique. We've helped clients achieve incredible purity levels for products like refined fish oils, vitamin E, and high-value specialty chemicals using our molecular distillation equipment.
When should I choose molecular distillation over other vacuum methods?
Are you unsure which distillation technique is the right investment for your valuable product? Making the wrong choice can lead to product loss or impurity. Let's clarify when molecular distillation truly shines.
You should choose molecular distillation for highly heat-sensitive, high molecular weight, or very viscous materials. Examples include valuable oils, waxes, vitamins, and specific pharmaceutical compounds where other vacuum methods might still cause degradation or be inefficient.

Deciding on the right distillation method is a critical step. It depends heavily on the properties of the material you are working with. As a company that provides a wide range of distillation solutions, including rotary evaporators, glass reactors, and advanced molecular distillation systems, we guide our customers through this decision process.
Here's a More Detailed Comparison:
| Feature | Standard Vacuum Distillation | Short-Path Distillation (Non-Molecular) | Molecular Distillation (Our Specialty) |
|---|---|---|---|
| Operating Pressure | Moderate (e.g., 1-100 mbar) | Lower (e.g., 0.01-1 mbar) | Extremely Low (e.g., <0.001 to 0.01 mbar) |
| Evaporator-Condenser Distance | Relatively Long (often involves a column) | Short (condenser close to evaporator, but external) | Very Short (internal condenser, few centimeters) |
| Typical Temperature | Reduced, but still can be significant | Lower than standard vacuum | Lowest possible for evaporation |
| Suitability for Molecular Weight | Low to moderate | Moderate to high | High to very high |
| Thermal Sensitivity of Material | For moderately sensitive materials | For sensitive materials | For extremely sensitive materials |
| Viscosity Handling | Low viscosity | Low to moderate viscosity | Moderate to high viscosity (especially with wiped film) |
| Primary Applications | Solvent recovery, basic purification of less sensitive compounds. | Fine chemical purification, some essential oils, compounds needing lower temps than standard vacuum. | Purification of Omega-3 fatty acids, Vitamin E (tocopherols), cannabinoids (CBD/THC), fragrances, plasticizers, monoglycerides. |
I recall a client in the natural products industry. They were trying to isolate a delicate active compound from a plant extract. Standard vacuum distillation, even short-path, was causing some degradation and loss of potency. When they moved to one of our molecular distillation systems, the results were remarkable. They achieved higher purity, better yield, and preserved the compound's activity. This is because molecular distillation minimizes the time the material spends at high temperatures and reduces the chance of thermal decomposition. If your material fits the profile of being very heat-sensitive, having a high molecular weight, or being quite viscous, then exploring molecular distillation with us is a very good idea. Our 16 years of export experience means we've seen a vast array of applications and can help tailor a solution.
Conclusion
Molecular distillation is a powerful, specialized form of vacuum distillation. It offers unique advantages for purifying highly sensitive, high-value compounds. Understanding this helps you choose wisely.
